Health ministry lists precautions and contraindications for COVID-19 vaccination
In a letter to all states and federal territories, the ministry emphasized that under the Emergency Use Permit, vaccination against the Coronavirus is indicated
- by B2B Desk 2021-01-15 06:01:27
The Health Ministry said Thursday that the interchangeability of COVID-19 vaccines is not allowed and that pregnant and lactating women should not receive the vaccines because they have not been part of any clinical trial of a vaccine so far. coronavirus.
In a letter to all states and federal territories, the ministry emphasized that under the Emergency Use Permit, vaccination against the Coronavirus is indicated only for those over 18 years of age. If necessary, the COVID-19 vaccine and other vaccines should be separated with an interval of at least 14 days.
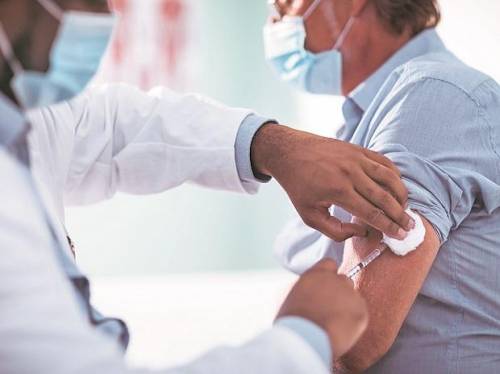
"The possibility of exchanging COVID-19 vaccines is not allowed. The second dose of the COVID-19 vaccine must be the same as the first," says the letter, written by Manohar Anani, additional secretary of the Trade Union Ministry.
The Ministry has listed the precautions and contraindications for COVID-19 vaccination, along with a comparative data sheet for each of the two vaccines (Covishield and Covaxine), which contains information on the vaccine platform, physical specifications, doses, Cold chain storage requirements, contraindications, and minor adverse (adverse) effects. Next immunization event).
He said this information should be shared with program managers at all levels and with cold chain processors and vaccinators for ease of reference.
In the list of contraindications, the Ministry of Health has warned against administering the vaccine to people who have a history of allergy or allergic reaction to a previous dose of the COVID-19 vaccine, and to people who suffer from immediate or delayed anaphylaxis or allergic reaction to vaccines or injectable treatments and pharmaceuticals. And food and more.
According to the letter, if there are active symptoms of SARS-CoV-2 infection, coronavirus patients who have received anti-SARS-CoV-2 monoclonal antibodies or convalescent plasma, and patients who are very ill and hospitalized for any disease , should Postpone COVID-19 vaccination for four to eight weeks after recovery.
"Pregnant and lactating women have not yet been part of any COVID-19 vaccine clinical trial. Therefore, pregnant or unsafe women who are pregnant and breastfeeding should not receive the COVID-19 vaccine at this time. "he declared.
The vaccine should be administered with caution to people with a history of any bleeding or clotting disorders (such as a deficiency of a clotting factor, a blood clotting disorder, or a platelet disorder, she said.
The following conditions are not contraindicated for COVID-19 vaccines: people with a history of SARS-CoV-2 infection and/or RT-PCR positive disease, a history of chronic disease, morbidity, immunodeficiency, HIV, and patients with immunosuppression due to In any case ".
Prime Minister Narendra Modi will launch the massive COVID-19 vaccination campaign in India on January 16 via video conference, even as appropriate doses of Covaxin vaccines from Bharat Biotech and Serum's Covishield Institute have been delivered across the country to all states. and union territories.
The letter also mentions mild post-vaccination effects after vaccination for both vaccines.
In the case of Covishied, some mild reversible effects such as injection site pain, injection site pain, headache, fatigue, muscle pain, malaise, pyrexia, chills, arthralgia and nausea can occur. Some of the mild airway effects of Covaxin include injection site pain, headache, fatigue, fever, body aches, abdominal pain, nausea and vomiting, dizziness, dizziness, tremors, sweating, cold, cough and swelling at the injection site. The letter added that acetaminophen can be used to relieve symptoms of adverse reactions after vaccination.
Also Read: 20 profit-making small businesses you can start with as low as Rs 20,000
POPULAR POSTS
Loan EMIs to Drop as RBI Slashes Repo Rate - Full MPC December 2025 Highlights
by Shan, 2025-12-05 11:49:44
Zoho Mail vs Gmail (2025): Which Email Platform Is Best for Businesses, Startups, and Students?
by Shan, 2025-10-09 12:17:26
PM Modi Launches GST Bachat Utsav: Lower Taxes, More Savings for Every Indian Household
by Shan, 2025-09-24 12:20:59
$100K H-1B Visa Fee Explained: Trump’s New Rule, Clarifications & Impact on Indian Tech Workers
by Shan, 2025-09-22 10:11:03
India-US Trade Deal Soon? Chief US Negotiator Arrives in Delhi as Talks Set to Begin Tomorrow
by Shan, 2025-09-15 11:54:28
Modi Meets Xi: Trump’s Tariffs, Strategic Autonomy, and the Future of Asia’s Power Balance
by Shan, 2025-09-03 06:40:06
Google Claims Gemini AI Uses Just ‘Five Drops of Water’ Per Prompt, Sparks Debate
by Shan, 2025-08-22 12:34:27
RECENTLY PUBLISHED

Pine Labs IPO 2025: Listing Date, Grey Market Premium, and Expert Outlook
- by Shan, 2025-11-05 09:57:07

The Agentic Revolution: Why Salesforce Is Betting Its Future on AI Agents
- by Shan, 2025-11-05 10:29:23

Top 10 Insurance Companies in India 2026: Life, Health, and General Insurance Leaders Explained
- by Shan, 2025-10-30 10:06:42

OpenAI Offers ChatGPT Go Free in India: What’s Behind This Big AI Giveaway?
- by Shan, 2025-10-28 12:19:11

Best Silver Investment Platforms for 2025: From CFDs to Digital Vaults Explained
- by Shan, 2025-10-23 12:22:46




 Subscribe now
Subscribe now 