If a coronavirus vaccine is developed, what will be the next challenges?
"This is the biggest logistical challenge the world has ever faced," said Toby Peters, an engineering and technology expert at the University of Birmingham. "We
- by B2B Desk 2020-06-26 10:48:29
Developing a COVID-19 vaccine in record time will be difficult. Producing enough to end the pandemic would be the greatest medical-industrial advance in history.
This work is underway.
From deploying experts amid global travel restrictions, managing extreme storage conditions, and even inventing new types of vials and syringes for billions of doses, the path is strewn with formidable hurdles, according to Reuters interviews with more than a dozen vaccine developers and their supporters.
Any hitch in an untested supply chain, which can extend from Pune in India to Oxford and England, Baltimore in the United States, can blow up or delay the complex process.
Col. Nelson Michael, director of the US Army's Infectious Diseases Research Center, which is working on the government's " Warp Speed" project to offer a large-scale vaccine in January, said that companies generally have years to determine these things.
"Now, they have weeks."
Much of the world's attention is focused on the scientific race to develop a COVID-19 vaccine. But behind the scenes, experts face a stark reality: We simply don't have the ability to manufacture, package, and distribute billions of doses at once.
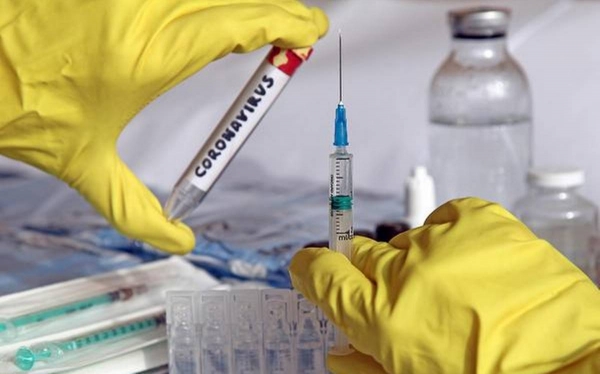
Companies and governments are competing to expand the machinery to address the severe shortage of automatic filling and finishing capacity, the final step in the manufacturing process of placing the vaccine in vials or syringes, closing, and packing for shipment.
"This is the biggest logistical challenge the world has ever faced," said Toby Peters, an engineering and technology expert at the University of Birmingham. "We could consider vaccinating 60% of the population."
Many developers, including frontrunner Moderna, are experimenting with new ways to alleviate the severe cold storage requirements for their vaccines, which they must now maintain at 80 ° C (-112 ° F).
SiO2 Materials Science produces vials that do not decompose in very cold temperatures.
Meanwhile, travel restrictions pose more general problems; Johnson & Johnson, which plans to start clinical trials this summer, has struggled to send vaccine experts to oversee the launch of production sites, for example.
'NEVER IN HISTORY'
By conducting massive clinical trials involving 10,000 to 30,000 volunteers per vaccine, the scientists hope to get an answer on whether the vaccine is working as early as this October. But even if they do succeed, mass manufacturing, motivating regulators to register and pack billions of doses is a big challenge.
In fact, the world is unlikely to go straight from a lack of vaccines at doses sufficient for everyone, in fact, said Seth Berkeley, CEO of the Global Alliance for Vaccine and Immunization Alliance.
"It is probably a personalized approach to start with," he said in an interview. "We are looking to have approximately a billion to two billion doses of vaccine in the first year, spread across the world population."
J&J partnered with the United States government on a $ 1 billion investment to accelerate the development and production of its vaccine, even before its success was proven. It has contracted Emergent Biosolutions and Catalent to manufacture large quantities in the United States. Catalent will also do some filling and finishing work.
"Not many vaccines have been developed in history at the same time, so this capacity does not exist," said Paul Stoffels, chief scientific officer at J&J, who sees fill capacity as the main limiting factor.
The emerging plant in Bayview, Maryland, can accommodate four vaccines in parallel using different platforms and manufacturing equipment.
Funded by the government in 2012, the plant includes disposable bioreactor reactors that feature plastic bags instead of stainless steel fermentation equipment, making it easy to switch from one vaccine to another.
"The company has received an additional $ 628 million to provide these four pavilions to support any candidate chosen by the government," Chief Executive Bob Kramer told Reuters this month.
BLOW-FILL-SEAL-REPEAT
In addition to working with J&J, New Jersey-based Catalent signed an agreement with British pharmaceutical company AstraZeneca last week to provide vial-filling and packaging services at its plant in Anagni, Italy. Its goal is to manage hundreds of millions of doses, starting in August 2020 and possibly until March 2022.
She ordered high-speed bottle filling equipment to increase production at the Indiana plant, where she also hires an additional 300 workers.
Her biggest challenge, Michael Riley, Catalan biology chief for North America, told Reuters she was trying to squeeze out the work that usually takes years or months.
Added to the challenge are the few glass bottles.
To save the glass, companies plan to use vials of more than five to 20 doses, but this poses new problems, such as possible waste, if not all doses are used before the vaccine breaks down.
"The downside is that after a healthcare professional opens a bottle, they need to vaccinate 20 people in no time, 24 hours," said Prashant Yadav, an expert in the global healthcare supply chain at the Center for Global Development in Washington.
As part of the same drive, the US Department of Health and Human Services and the Department of Defense awarded ApiJect systems up to $ 138 million to upgrade their facilities to produce up to 100 million pre-filled plastic syringes for the end of this year and up to 600 million in 2021.
The company plans to use a technology called Blow-Fill-Seal, in which the syringe is inflated with plastic, filled with vaccines, and sealed in seconds. This will need approval from the Food and Drug Administration, "CEO Jay Walker told Reuters.
Also Read: Fitch upgrades Reliance industries’ long-term local currency IDR to BBB+
POPULAR POSTS
How a Free AI Visibility Tool Helps Businesses Grow in the AI Driven Search Era
by Aakash Ladha , 2026-03-06 10:40:04
Loan EMIs to Drop as RBI Slashes Repo Rate - Full MPC December 2025 Highlights
by Shan, 2025-12-05 11:49:44
Zoho Mail vs Gmail (2025): Which Email Platform Is Best for Businesses, Startups, and Students?
by Shan, 2025-10-09 12:17:26
PM Modi Launches GST Bachat Utsav: Lower Taxes, More Savings for Every Indian Household
by Shan, 2025-09-24 12:20:59
$100K H-1B Visa Fee Explained: Trump’s New Rule, Clarifications & Impact on Indian Tech Workers
by Shan, 2025-09-22 10:11:03
India-US Trade Deal Soon? Chief US Negotiator Arrives in Delhi as Talks Set to Begin Tomorrow
by Shan, 2025-09-15 11:54:28
Modi Meets Xi: Trump’s Tariffs, Strategic Autonomy, and the Future of Asia’s Power Balance
by Shan, 2025-09-03 06:40:06
RECENTLY PUBLISHED

Pine Labs IPO 2025: Listing Date, Grey Market Premium, and Expert Outlook
- by Shan, 2025-11-05 09:57:07

The Agentic Revolution: Why Salesforce Is Betting Its Future on AI Agents
- by Shan, 2025-11-05 10:29:23

Top 10 Insurance Companies in India 2026: Life, Health, and General Insurance Leaders Explained
- by Shan, 2025-10-30 10:06:42

OpenAI Offers ChatGPT Go Free in India: What’s Behind This Big AI Giveaway?
- by Shan, 2025-10-28 12:19:11

Best Silver Investment Platforms for 2025: From CFDs to Digital Vaults Explained
- by Shan, 2025-10-23 12:22:46




 Subscribe now
Subscribe now 